Elon Musk is one of those people who only invests in world-changing projects. (Well, maybe not Twitter.) One such company is Neuralink. Previously, we saw it successfully test a brain implant on monkeys. At the end of 2022, the company announced that it would start testing a similar implant on humans in 2023. But we know that the FDA hasn’t approved the use of the same devices that killed all the pigs on which they were previously tested.
The FDA Doesn’t Believe That Brain Implants Are Safe
“The agency’s main safety concerns related to the device’s lithium battery, the potential for the implant’s tiny wires to migrate to other areas of the brain, and whether and how the device could be removed without damaging brain tissue,” current and former Neuralink employees told Reuters.
In simple terms, the FDA doesn’t think the battery system will work properly. To prove otherwise, Neuralink would have to provide more evidence.
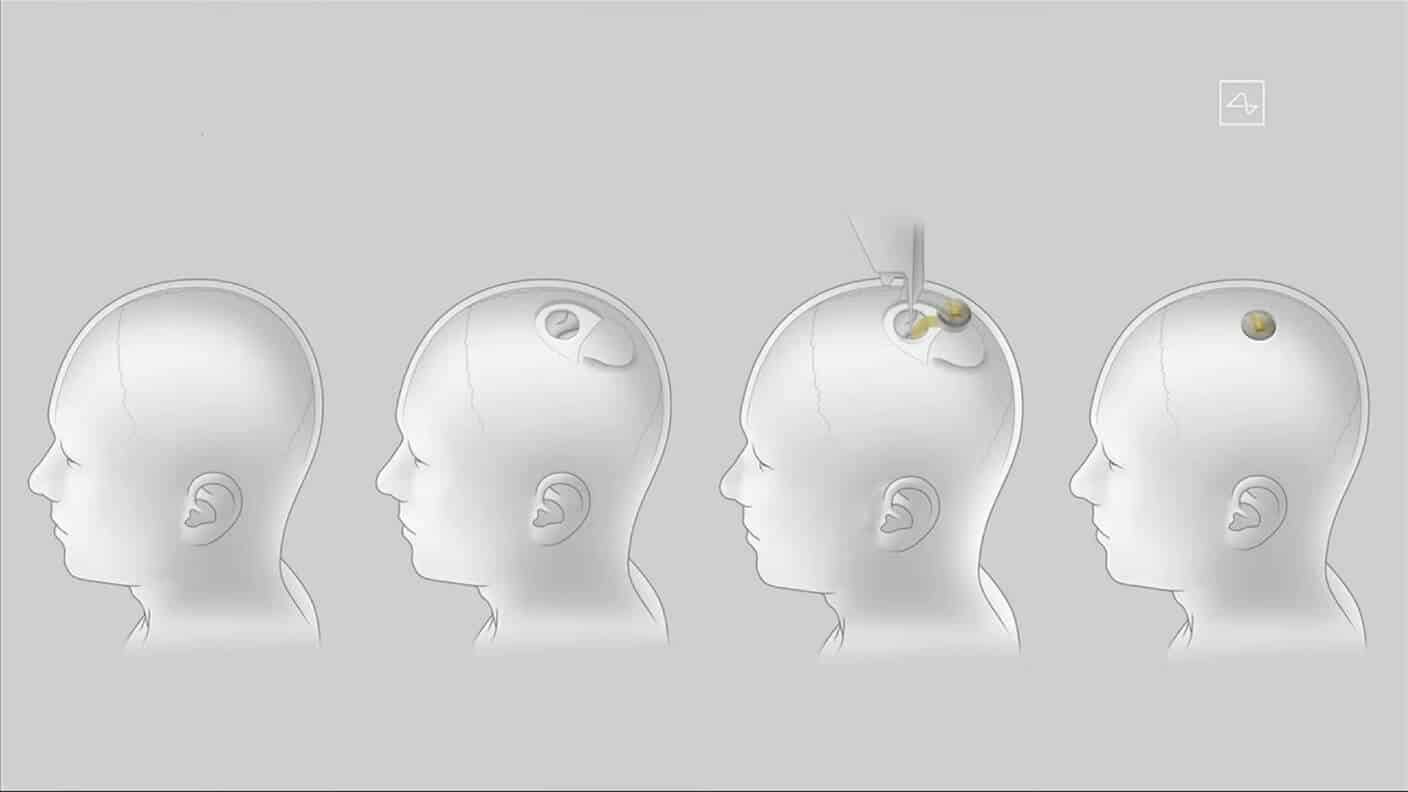
Another thing that keeps the FDA on its toes is the potential problems that could arise if there is a need for the removal or upgrade of brain implants. The reason is that the brain implant has tiny electrical leads that reach into the patient’s grey matter. They are so small that there is a risk that they could break off during removal. The FDA has similar concerns about regular use.
In November last year, Elon Musk claimed that they would have FDA approval “within six months.” So if Neuralink gets permission, the first products could be on the market before 2030.
We should remember previous words to remind you how serious Elon Musk is about this project. He said he wouldn’t mind implanting a Neuralink brain chip in one of his children. If one of his children has a serious injury, he would implant the chip in their body.





