Neuralink has received approval from the U.S. Food and Drug Administration (FDA) to conduct human trials. The company has previously tested its brain-to-computer connection system on animals, including monkeys. In one test, a monkey was able to control a computer cursor with its mind. Now, Neuralink is moving on to human trials. The company has not yet announced when or where the trials will take place. Or perhaps who will be eligible to participate. However, the approval from the FDA is a major milestone for Neuralink. It brings the company one step closer to its goal of developing a BCI that can be used to treat a variety of neurological disorders.
Neuralink receives FDA approval for Human testing
Neuralink is a neurotechnology company that develops implantable brain-computer interfaces (BCIs). The company was founded in 2016 by Elon Musk and a team of scientists and engineers. Neuralink's goal is to create a BCI that can be used to treat a variety of neurological disorders. Including paralysis, blindness, and hearing loss. The company has also expressed interest in developing BCIs that can be used to enhance human cognition and performance.
In a major step forward, Neuralink recently received a green light from FDA to begin human trials of its BCI technology. “This is the result of incredible work by the Neuralink team in close collaboration with the FDA. It represents an important first step that will one day allow our technology to help many people,” the company wrote in a tweet.
Elon Musk said in December that Neuralink will be able to implant its first brain chip in a human in six months. And the FDA approval is in line with Musk's prediction. At a Neuralink event in December, Musk showed off brain chips that had been implanted in monkeys and pigs. He said the chips could be used to restore vision or motor function in people with paralysis or blindness.
Will you register for Neuralink trails?
Many of you might not know it, but Neuralink applied for FDA approval before. However, its appeal was rejected due to safety concerns. Otherwise, it would’ve gained approval for human trials in 2022. The company has made significant progress since then, and the recent approval is a sign of that hard work. Also, it is the only company that has managed to clinch the FDA’s final seal of approval for human trials to date.
Neuralink has not yet announced plans to recruit participants for the trials. But the company seems eager to make its technology accessible to a wide range of individuals in the future. So if you’re interested in learning whether you may qualify for future Neuralink clinical trials, you can join their Patient Registry.
How will Neuralink BCI work on humans?
BCI technology has been studied by scientists for decades. Several companies have developed promising systems. But none have received FDA approval until Neuralink.
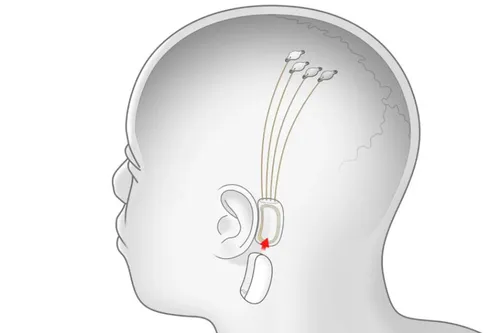
Now many of you might be curious about how this BCI system or tech will work on humans. First off, Neuralink's BCI requires invasive brain surgery. The patient will then be implanted with a system called Link. Link is a small, circular device that processes and translates neural signals.

It will then be connected to a series of thin, flexible threads that will be inserted into the brain tissue. These threads are the ones that will detect neural signals and send them to the Link. The Link will process and translate them into commands.
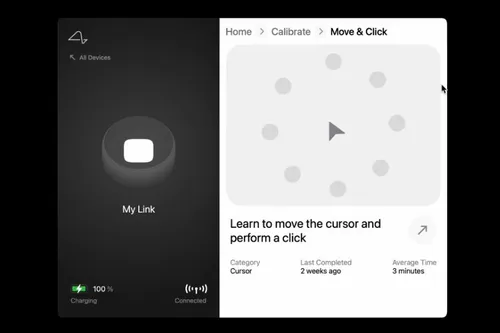
Patients with Neuralink devices will also need to learn to control them. Fortunately, there's a Neuralink app for it. The app will allow patients to control external devices, such as mice and keyboards, through a Bluetooth connection.
Neuralink's BCI technology has the potential to revolutionize the way we interact with the world. It could allow people with disabilities to regain their mobility and independence, and it could even be used to create new forms of entertainment and communication.
Loading